Oncogenes and Cancer
Oncogenes are important classes, or groups, of genetic mutations that cause cancer. They’re mutated (changed) forms of genes that control how cells grow and divide. Research shows specific oncogenes d
What are oncogenes?
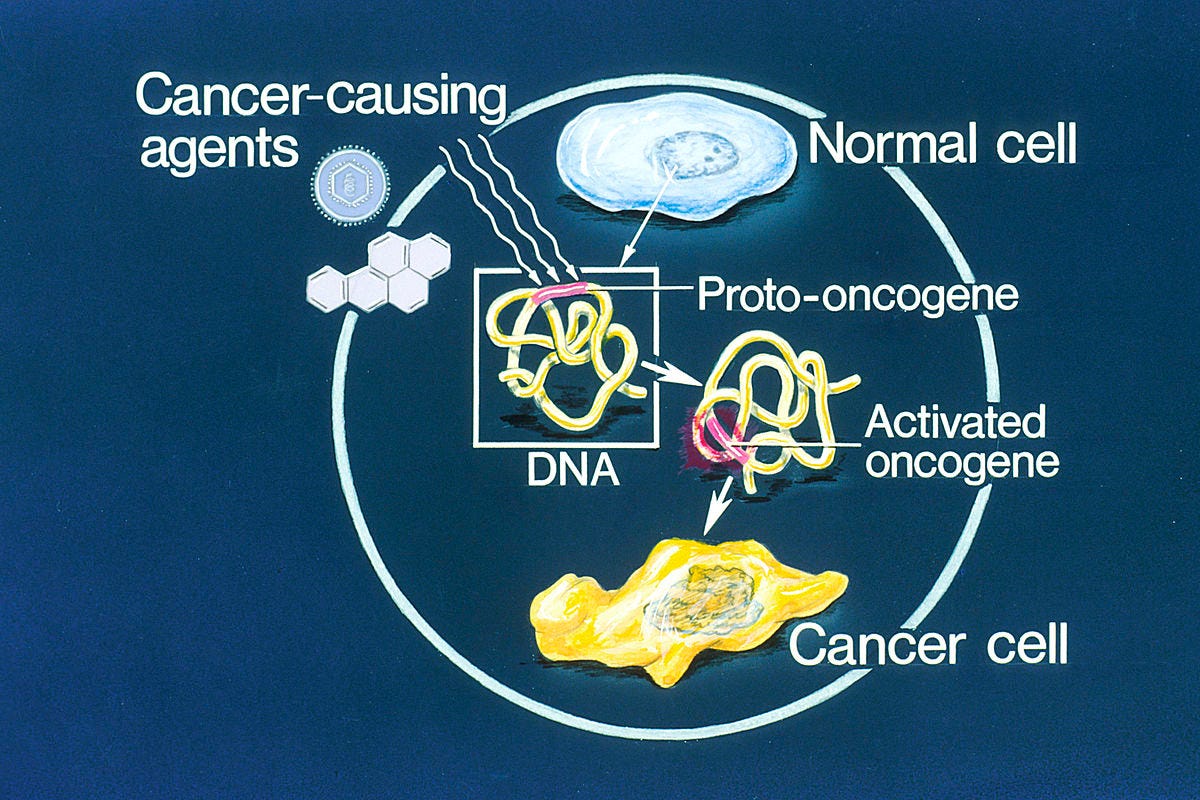
An oncogene is a gene that may cause cancer. It’s a mutated (changed) form of a normal gene, called a proto-oncogene, that manages cell growth. When proto-oncogenes mutate into oncogenes, they cause cells to grow and divide uncontrollably. Eventually, the abnormal cells may form tumors. Think of it this way: Oncogenic means “causing tumor growth.” And this is what oncogenes do.
Understanding how oncogenes drive tumor growth helps healthcare providers develop treatments targeting oncogenes. It helps them treat the cancers that oncogenes cause.
Types of oncogenes
Providers have linked more than 100 different oncogenes to various kinds of cancer. For example, approximately 1in 5 cancers involve various forms of Ras genes. Ras genes make proteins that manage how cells receive signals, grow, and die.
Other oncogenes are associated with specific kinds of cancer, including:
BCR/ABL1 gene in chronic myeloid leukemia and some types of B-cell acute lymphocytic leukemia
CMYC gene in Burkitt lymphoma
EGFR and EML4AK genes in adenocarcinoma of the lung
HER2 gene in breast cancer
KRAS gene in pancreatic cancer, colon cancer, and lung cancer
NMYC gene in small cell lung cancer and neuroblastoma
Function
How do oncogenes work?
To understand how oncogenes work, you need to know about proto-oncogenes. Proto-oncogenes are normal genes that may mutate and become oncogenes.
Proto-oncogenes are genes that normally help cells grow and divide to make new cells or to help cells stay alive. When a proto-oncogene mutates (changes) or there are too many copies of it, it can become turned on (activated) when it is not supposed to be, at which point it's now called an oncogene. When this happens, the cell can start to grow out of control, which might lead to cancer.
A proto-oncogene normally functions in a way much like the gas pedal on a car. It helps the cell grow and divide. An oncogene is like a gas pedal that is stuck down, which causes the cell to divide out of control.
Oncogenes can be turned on (activated) in cells in different ways. For example:
Gene variants/mutations: Some people have differences in the ‘code’ of their genes that can cause an oncogene to be turned on all the time. These types of gene changes can be inherited from a parent, or they can occur during a person’s life, when a mistake is made when copying the gene during cell division.
Epigenetic changes: Cells normally have ways of turning genes on or off that don’t involve changes in the genes themselves. Instead, different chemical groups can be attached to genetic material (DNA or RNA) that affect whether a gene is turned on. These types of epigenetic changes can sometimes lead to an oncogene being turned on.
Chromosome rearrangements: Chromosomes are long strands of DNA in each cell that contain genes. Sometimes when a cell is dividing, the sequence of the DNA in a chromosome can be changed. This might put a gene that functions as a type of ‘on’ switch next to a proto-oncogene, keeping this gene turned on even when it shouldn’t be. This new oncogene can result in the cell growing out of control.
Gene duplication: Some cells have extra copies of a gene, which might lead to them making too much of a certain protein.
A small number of family cancer syndromes are linked to an inherited change in an oncogene. These types of changes can sometimes be the first step in a cell becoming a cancer cell. But most changes involving oncogenes are acquired during a person’s lifetime, rather than being inherited.
Tumor suppressor genes
Tumor suppressor genes are normal genes that slow down cell division or tell cells to die at the right time (a process known as apoptosis or programmed cell death). When tumor suppressor genes don't work properly, cells can grow out of control, which can lead to cancer.
A tumor suppressor gene is like the brake pedal on a car. It normally helps keep the cell from dividing too quickly, just as a brake keeps a car from going too fast. When something goes wrong with a tumor suppressor gene, such as a pathogenic variant (mutation) that stops it from working, cell division can get out of control.
Inherited changes in tumor suppressor genes have been found in some family cancer syndromes. They cause certain types of cancer to run in families. But most tumor suppressor gene mutations are acquired during a person's lifetime, not inherited.
For example, TP53 is an important tumor suppressor gene. It codes for the p53 protein, which helps keep cell division under control. Inherited changes in the TP53 gene can lead to Li-Fraumeni syndrome. Family members with this syndrome have an increased risk of several types of cancer because all of their cells have this TP53 gene change.
Changes in the TP53 gene are also very common in cancer cells in people without an inherited cancer syndrome. These TP53 changes are acquired during the person’s life. These changes can help the cancer cells grow, but they are found only in the cancer cells, not in other cells in the body, so they can’t be passed on to a person’s children.
DNA repair genes
When a cell divides to make new cells, it needs to make a new copy of all of its DNA. This is a complex process, and sometimes it results in mistakes in the DNA.
Genes known as DNA repair genes act like a person who repairs a car. They help fix mistakes in the DNA, or if they can’t fix them, they trigger the cell to die so the mistakes can’t cause any further problems.
When something goes wrong with one of these DNA repair genes, it can allow more mistakes to build up inside the cell. Some of these might affect other genes, which could lead to the cell growing out of control.
As with other types of gene changes, changes in DNA repair genes can either be inherited from a parent or acquired during a person’s lifetime.
Examples of DNA repair genes include the BRCA1 and BRCA2 genes. People who inherit a pathogenic variant (mutation) in one of these genes have a higher risk of some types of cancer, particularly breast and ovarian cancer among women. (For more information, see Family Cancer Syndromes.) But changes in these genes are also sometimes seen in tumor cells in people who did not inherit one of these mutations.
What triggers proto-oncogenes to mutate?
Providers aren’t sure why oncogenes form, but many things that cause cancer likely play a role. This includes overexposure to sunlight or cancer-causing substances (carcinogens). Certain viral infections may also play a role.
Instead of being a genetic mutation you inherit, most oncogenes develop during a person’s lifetime.
Proto-oncogenes mutate in different ways:
Point mutation. Cells copy their DNA (which contains genes) when they’re ready to divide and make new cells. But sometimes, a change, addition, or deletion happens in the DNA, causing a proto-oncogene to become an oncogene.
Gene amplification. Sometimes, a chromosome (the structure that carries DNA) has too many copies of a certain gene.
Chromosomal rearrangement. Sometimes, a piece of one chromosome breaks off and swaps places with another chromosome. This process, called translocation, can create an oncogene.
Sometimes, oncogenes and mutated tumor suppressor genes work together to cause cancer. Tumor suppressor genes are another class of genes that may cause cancer.
Care
Why are oncogenes important to cancer treatment?
Cancer typically happens when several genes mutate. For example, most cancerous tumors show signs of 30 to 60 different genetic mutations. But oncogenes are powerful. In some cases, a single oncogene may trigger cell growth that leads to cancer.
But there’s a potential upside. It’s easier for healthcare providers to target one genetic mutation than many mutations, making cancer treatment more effective.
For instance, they know chronic myelogenous leukemia (CML) happens when a single type of proto-oncogene mutates and becomes the BCR-ABL oncogene. The oncogene makes abnormal enzymes that let abnormal white blood cells multiply uncontrollably.
Tyrosine kinase inhibitor (TKI) drugs block abnormal BCR-ABL enzymes, so the abnormal white blood cells die. This puts CML into remission. Remission means you don’t have signs or symptoms of cancer. Before this treatment, only about 1 in 5 people with CML were alive five years after diagnosis. But now, people are living significantly longer because of drugs designed to target the oncogene.
Additional Common Questions
Is p53 an oncogene?
No, p53 is a tumor suppressor gene. But like an oncogene, it can lead to cancer. Tumor suppressor genes tell cells when to stop making more cells. But if a tumor suppressor gene mutates, it can no longer send the “stop” signal. The cells can multiply out of control and form tumors.
Consider this:
Cancer is a genetic disease
Gain of oncogene(s) + Loss of TSG = CANCER
Oncogenes
The term “oncogene” was coined in 1969 by R. Huebner & G. Todaro
• Genes that have the potential to cause cancer (proto-oncogenes)
• Transform healthy cells – cause them to gain “hallmarks of cancer”
• First discovered in viruses, later in cells
Src – the first oncogene
• The first oncogene was discovered before we understood what oncogenes are! Peyton Rous, 1911 – cell-free extract from chicken tumors can cause new tumors when injected into healthy chickens Rous Sarcoma – a form of cancer which infects chickens Rous Sarcoma Virus (RSV) – a retrovirus which generates a cDNA that inserts into the chickens’ DNA v-Src – the gene which gets expressed by RSV v-Src = oncogenic retrovirus.
Other viral oncogenes
• Rous Sarcoma
• Abelson leukemia
• Avian erythroblastosis
• McDonough feline sarcoma
• H-Z feline
• Murine sarcoma 3611
• Simian sarcoma
• Harvey sarcoma src abl erbB fms kit raf sis H-ras
Many oncogenes have been identified in viruses
• But… Most human cancers are not viral in origin
• Cellular versions of oncogenes = proto-oncogenes
Src in human tumors, Src expression/activity upregulated in:
• Breast ca
• Pancreatic ca
• Ovarian ca
• Head & Neck ca (My Cancer was Src)
• Lung ca
• Gastric ca
• Colon ca – C-terminal truncation identified
Scientists expected oncogenes to be genetically dominant
• BUT – fusion of cancer and normal cells creates non-tumor forming hybrid
Injected into mice produced no tumor
Oncogenes à cancer, end of story, right?!
NOPE!!!!
Conclusion Drawn
Genetic changes = Cancer
Oncogenes
• Gene amplification
• Insertion of powerful (viral) promoters
• Activating mutations
Tumor suppressors
• Gene deletion
• Silencing mutations
• Loss of heterozygosity
• Promoter hypermethylation







Good evening. What is the name of your book please and where is it available from please? Thank you